Description
InterOss® Syringe is a natural hydroxyapatite bone grafting material for use in dentistry. Made from a proven multi-step purification process which leaves only a bone composition, it is a highly purified osteoconductive material for bone regeneration.
Having an interconnected network of macro and micro pores and large inner surface areas that provides an ideal environment for cell attachment, InterOss® is chemically and structurally comparable to mineralized human bone.
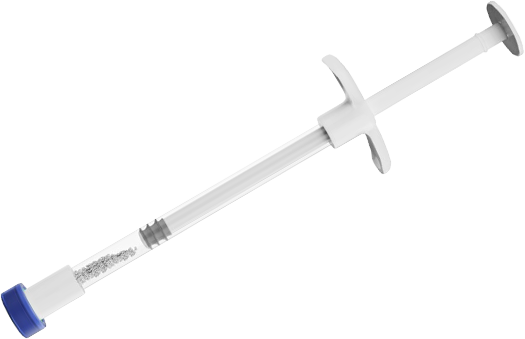
InterOss® Syringe is designed to deliver InterOss® granules more precisely to the intended treatment site without having to use sterile instruments. It is available in sterilized granule form and is dedicated for single uses (also available in a vial).

Description
InterOss® Syringe is a natural hydroxyapatite bone grafting material for use in dentistry. Made from a proven multi-step purification process which leaves only a bone composition, it is a highly purified osteoconductive material for bone regeneration.
Having an interconnected network of macro and micro pores and large inner surface areas that provides an ideal environment for cell attachment, InterOss® is chemically and structurally comparable to mineralized human bone.
InterOss® Syringe is designed to deliver InterOss® granules more precisely to the intended treatment site without having to use sterile instruments. It is available in sterilized granule form and is dedicated for single uses (also available in a vial).
Product Specifications
Small Granules
(0.25 – 1.0 mm)
| REF | Volume |
|---|---|
| IOSGS025 | 0.25 cc |
| IOSGS050 | 0.5 cc |
| IOSGS100 | 1.0 cc |
Large Granules
(1.0 – 2.0 mm)
| REF | Volume |
|---|---|
| IOLGS050 | 0.5 cc |
| IOLGS100 | 1.0 cc |
Indications for Use
InterOss® is recommended for:
- Filling of peri-implant defects in conjunction with products intended for guided bone regeneration (GBR)
- Filling of periodontal defects in conjunction with products intended for guided tissue regeneration (GTR) and guided bone regeneration (GBR)
- Reconstruction or augmentation of the alveolar ridge
- Filling of periodontal defects
- Filling of defects after root resection, apicectomy, and cystectomy
- Filling of extraction sockets to enhance preservation of the alveolar ridge
- Elevation of the maxillary sinus floor
Studies
InterOss® is 100% natural, biocompatible, highly purified, and has a large inner surface area, multi-porosity, and long term stability. It is validated and documented in clinical studies through universities in the US and abroad and case studies through clinicians involving procedures such as horizontal/vertical augmentation, sinus augmentation, and sinus elevation.
Downloads
Inquiry
InterOss® Syringe is cleared by the FDA and is registered in Europe, Middle East, Asia, and Latin America. If you are a distributor or clinician and have questions about the product, please submit an inquiry below.