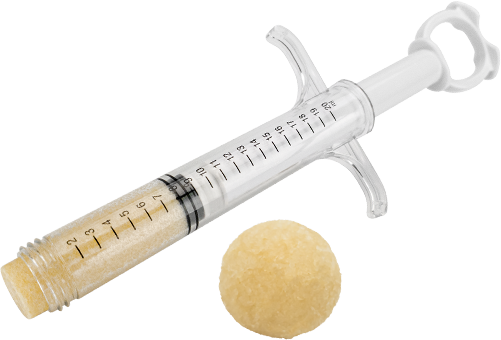
Description
AlloGen™ DBM is a putty made from human demineralized bone matrix (DBM) formulated with a biocompatible and bioresorbable carrier that is not easily broken down by bodily fluids or temperature, resulting in its resistance to irrigation. It possesses excellent handling and performance characteristics, and it can be easily molded into any shape and compressed into bone voids. AlloGen™ DBM is available in a syringe.

Description
AlloGen™ DBM is a putty made from human demineralized bone matrix (DBM) formulated with a biocompatible and bioresorbable carrier that is not easily broken down by bodily fluids or temperature, resulting in its resistance to irrigation. It possesses excellent handling and performance characteristics, and it can be easily molded into any shape and compressed into bone voids. AlloGen™ DBM is available in a syringe.
Product Specifications
Available Options
| Volume |
|---|
| 1.0 cc, 2.5 cc, 5.0 cc, 10.0 cc |
Indications for Use
- Intended to be used for the filling of bony voids or gaps of the skeletal system e.g. the extremities, spine, pelvis, etc
- Intended for use as a bone void filler for voids or gaps that are not intrinsic to the stability of the bony structure
- Indicated for use in the treatment of surgically created osseous defects or osseous defects resulting from traumatic injury to the bone
- Should not be used to treat large defects that in the surgeon’s opinion would fail to heal spontaneously
Learn More
AlloGen™ DBM is currently not available for sale within the United States. Please check with a representative for further information and contact us below.